What creatures were the common ancestors of land plants?
※The content is current at the time of writing.
Elucidating common principles of plant body building at the genetic level
Research Overview

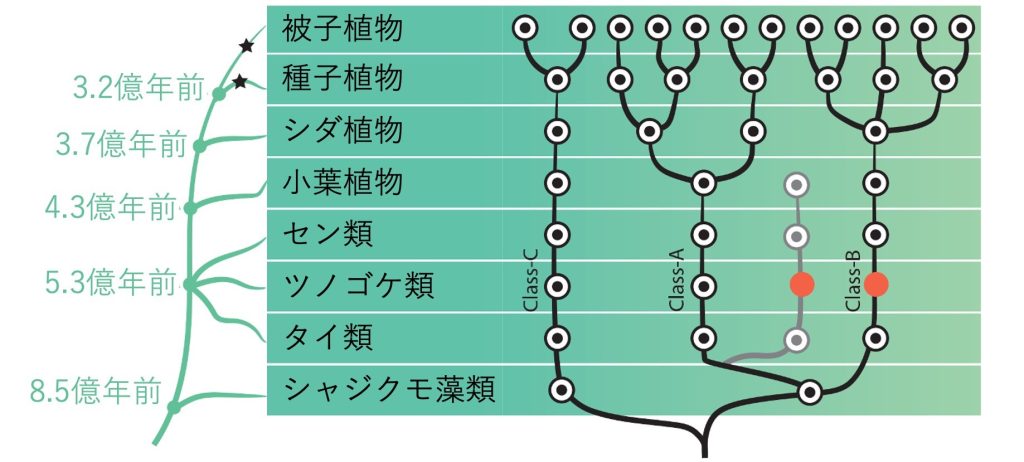
It is said that more than 300,000 species of terrestrial plants inhabit the earth, and that terrestrial plants account for about 80% of the biomass (weight of living organisms) on the earth. In addition to flowering angiosperms, terrestrial plants include gymnosperms such as cedar and pine, ferns and pinnipeds, which reproduce by spores rather than seeds, and mosses, which do not have vascular bundles. Recent molecular phylogenetic analysis has shown that all living land plants are derived from a single common ancestor that diverged from green algae about 500 million years ago. So what kind of organism was the common ancestor of land plants? Unfortunately, there is no direct way to answer this question, as no fossils have yet been discovered that would allow us to determine what plants looked like at that time.
However, the evolutionary history of living organisms is inscribed in their genes (DNA sequences), and it is possible to infer the characteristics of common ancestors by comparing the DNA sequences with the body building (development) mechanisms of the present-day species. In our laboratory, we are working to understand the developmental control mechanisms common to terrestrial plants by elucidating the developmental mechanisms at the genetic level, using the moss moss, which belongs to the moss phyla that separated from other phyla at the base of terrestrial plant evolution. In the past, we have focused on auxin, a type of plant hormone, and have analyzed the origin and evolution of auxin response mechanisms.
Research Features
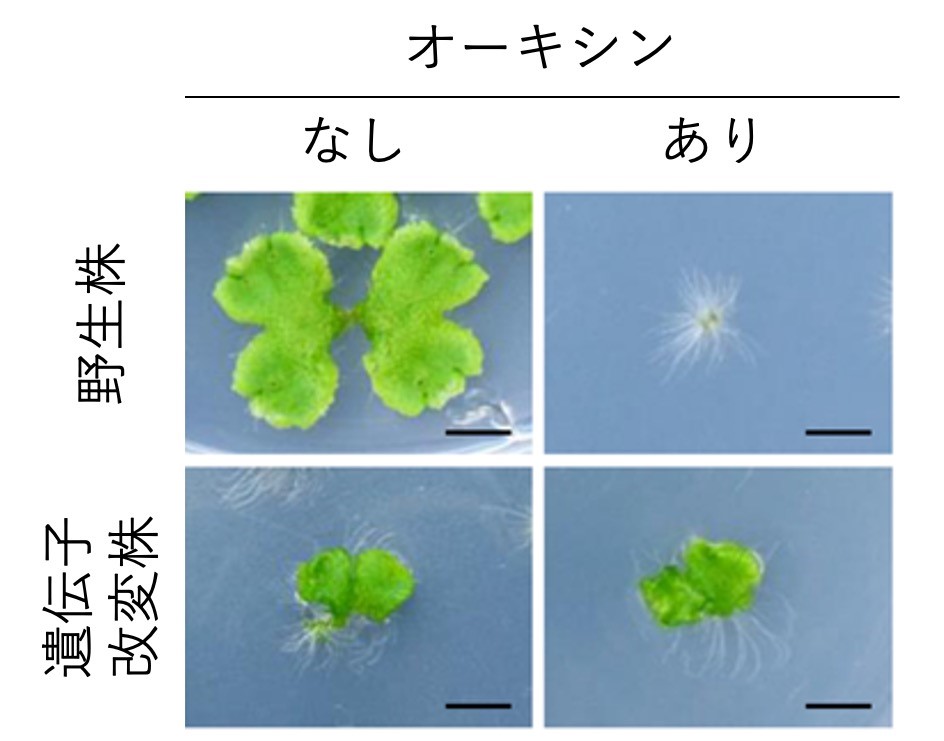
In angiosperms, auxin regulates development and growth throughout the life cycle, including organogenesis of roots, leaves, and flowers, vascular patterning, and bending response to light and gravity, and is widely used in agriculture as a herbicide and root promoter. Most of the plants used in agriculture and other industrial applications are angiosperms, and most of the auxin research in the past has been conducted on angiosperms. The results of these studies revealed that the auxin response is mainly mediated by the ARF protein, but the large number of genes involved in the auxin response in angiosperms (e.g., rice has 25 different ARF genes) has hindered research. The evolutionary question of when and how the auxin response mechanism was acquired is also an unresolved issue. The evolutionary question of how and when the auxin response mechanism was acquired remained unresolved. In 2008, we began our research using the Zeni moss (Fig. 1), which was being developed as a new experimental model at the time. In addition to its advantages such as fast growth, easy cultivation, and high efficiency of genetic recombination, preliminary analysis suggested that it had low genetic redundancy, and we thought it would be useful as a new model for auxin research.
Research Attraction
Our experiments using the moss model (Fig. 2) revealed that the moss has a common but minimal auxin response mechanism with only three ARF species in common with other angiosperms, and also revealed differences in the basic functions of the three ARF species. [1,2] This result means that the auxin response mechanism was already established in the common ancestor of land plants that lived 500 million years ago. Next, a comprehensive analysis using OneKP, a database of gene sequences of more than 1,000 plant species, revealed that ARF was first created by gene fusion in ancestral algae, and then the auxin receptor mechanism was retrofitted in the common ancestor of land plants as a switch that regulates ARF activity 3]. We also showed in experiments using multiple ferns and mosses that during land plant evolution, the auxin response mechanism began to become more complex in the common ancestor of ferns and seed plants (Figure 3), resulting in an increase in the type and amplitude of gene expression responses [3]. In this way, our research uses plant species that are alive today in our experiments, but by comparing the results among species, we can reach out to events that have occurred over hundreds of millions of years of evolution. People yearn for worlds that are out of their reach, such as outer space, the deep sea, and dinosaurs, and I believe that our research has the same romanticism as these fields.
[1] Kato H, et al. 2015. Plos Genetics ( https://doi.org/10.1371/journal.pgen.1005084 )
[2] Kato H, et al. 2020. Nature Plants (https://doi.org/10.1038/s41477-020-0662-y)
[3] Mutte SK, Kato H, et al. 2018. eLife ( https://doi.org/10.7554/eLife.33399 )
Future Outlook
Currently, I am working on several research themes in parallel based on my previous auxin research using Zeni mosses, one of which is the analysis of auxin-responsive genes common to land plants discovered through comparative analysis of mosses and ferns. The auxin response mechanism itself is nothing more than a switch that activates a specific group of genes, and I would like to understand the mechanism that changes the shape and physical and chemical properties of cells beyond the switch. Another theme is to understand the mechanism of formation of goblet bodies and asexual buds (Fig. 4), which are organs for asexual reproduction. These organs are unique to A. zeama and related species, but recent studies have shown that hormones common to land plants, including auxin, are involved in their regulation, and we expect that this will lead to the elucidation of the developmental control mechanisms common to land plants. We also believe that model organisms, including Zeni mosses, are specialized organisms that have evolved in the same way as other species, and that experiments using more plant species are needed to understand common principles. In the future, we would like to conduct experiments using small-leaved plants and ferns, which are divided between mosses and angiosperms.
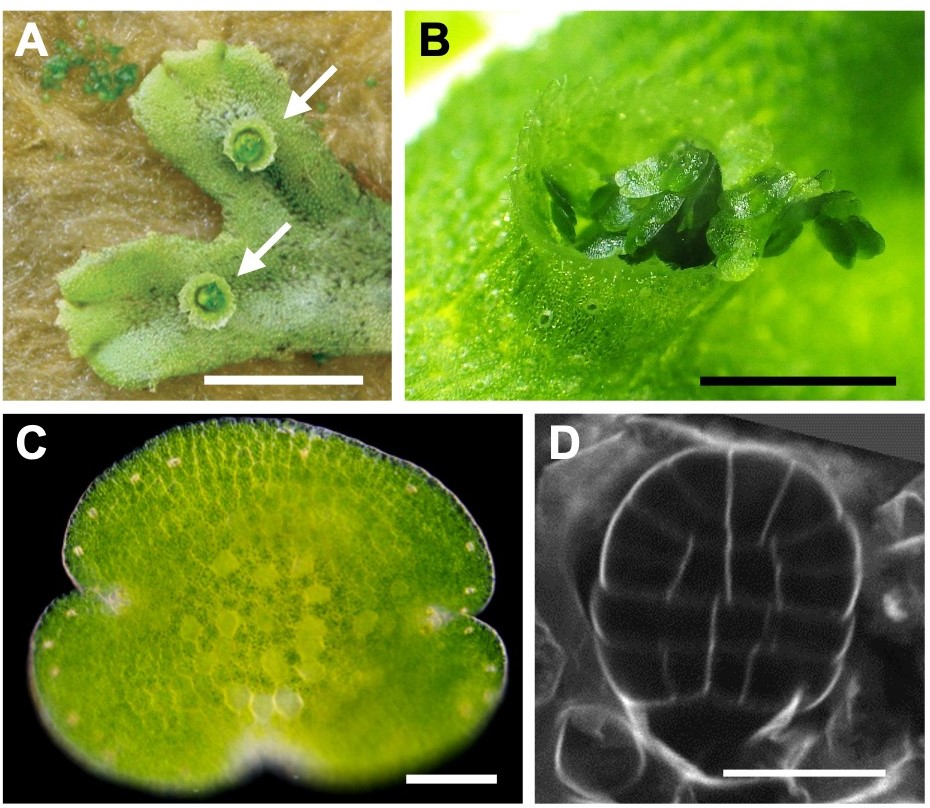
(A) Cup-shaped body formed on the back of the foliage (arrow)
(B) Close-up of the goblet body. Dark green objects are asexual buds.
(C) Close-up of asexual bud
(D) Fluorescence-stained photograph of cell wall of asexual buds in early developmental stages.
Bar=1cm (A), 2mm (B), 0.1mm (C), 20μm (D)
Message to those who are interested in this research
Research is an activity like climbing a large mountain of knowledge accumulated by our predecessors and building a new step on top of it. I believe that the primary driving force behind such research activities is the curiosity to see and learn about the unknown. With the recent development of sequencing technology, it is now possible to decode the genome (DNA sequence of all genes) of any organism, making it easier to study species that have been difficult to study at the genetic level. How is the shape of plants determined? How was this mechanism acquired and changed during evolution? or “I wonder about this part of my favorite plant,” with people who are passionate about these questions! I am looking forward to working together with people who are passionate about their favorite plants.
For more detailed research results, please visit our laboratory’s website.